🧪 Enzyme – AI-Powered Compliance Automation for Life Sciences
⚙️ Streamline FDA and ISO Compliance with AI Workflow Automation
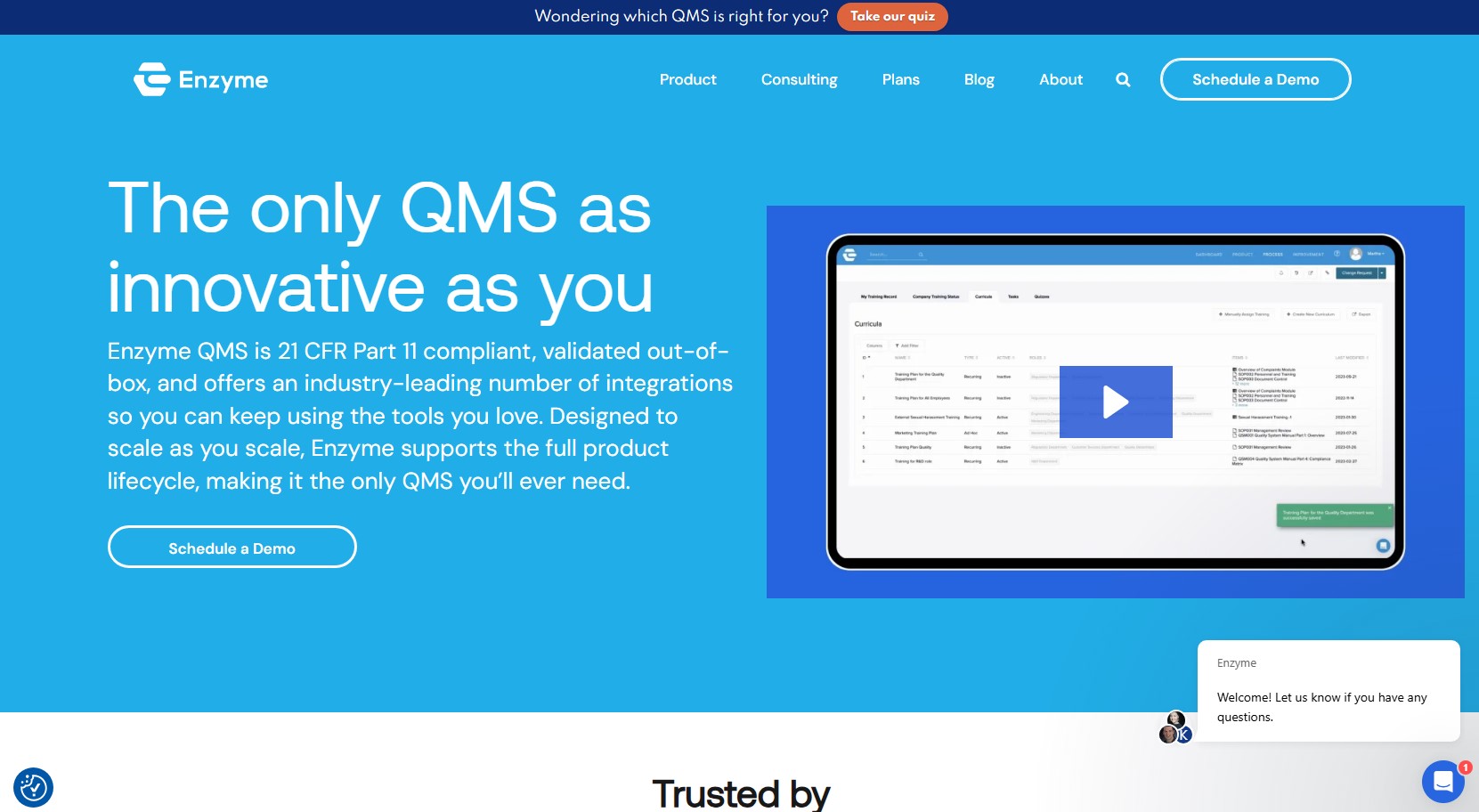
Enzyme is an AI-driven quality management system (QMS) tailored for life sciences companies aiming to automate and streamline FDA and ISO compliance. Purpose-built for biotech, medtech, and pharma teams, Enzyme integrates compliance requirements directly into day-to-day workflows, enabling faster product development with full regulatory alignment.
📄 Embedded QMS Designed for GxP Environments
Enzyme’s embedded QMS supports 21 CFR Part 11, ISO 13485, and ISO 14971 frameworks. It includes pre-configured workflows for design controls, risk management, document control, training records, and CAPAs. Every process is digitally traceable and audit-ready, reducing the burden of manual documentation.
🔍 Real-Time Audit Trails and Validation
The platform automatically logs user activity, timestamps, and version histories to maintain continuous validation and compliance. With built-in electronic signatures and controlled document approvals, teams can demonstrate readiness for FDA audits and ISO inspections at any time.
📂 Intelligent Document and Training Control
Enzyme centralizes document creation, review, and approval while ensuring version control and employee training status tracking. AI-backed reminders and workflows ensure that all documents stay current and that staff certifications remain audit-compliant.
🤝 Collaborative Design and Risk Management
Engineering, quality, and regulatory teams can work in real time to manage design inputs, outputs, and verifications. Enzyme’s AI-enhanced risk management module links design controls to hazards, mitigations, and validations, providing a complete risk traceability matrix.
🧬 Integrated Product Development Workflows
From concept to submission, Enzyme links design history files (DHF), device master records (DMR), and change controls in one environment. This ensures traceability across all design and development artifacts, supporting seamless transitions through FDA submission stages.
🚀 Rapid Implementation and Scalable Architecture
Enzyme offers guided onboarding and expert support to configure the platform for specific regulatory and operational needs. Its cloud-based architecture ensures secure access, scalability for growing teams, and seamless integration with tools like Jira, GitHub, and Slack.
🔐 FDA-Compliant Cloud Security
Built for regulated environments, Enzyme uses HIPAA-compliant hosting, data encryption, and regular penetration testing. The platform maintains full alignment with FDA cybersecurity expectations and best practices for software used in medical device development.
✅ Conclusion
Enzyme is a purpose-built AI QMS for life sciences companies that demand efficiency, traceability, and regulatory compliance. With automated design controls, audit-ready documentation, and intelligent risk management, Enzyme enables faster, safer product development while maintaining full FDA and ISO compliance.
Keywords: Enzyme AI QMS, life sciences compliance tool, FDA compliance automation, ISO 13485 software, risk management for medtech, AI-powered QMS for biotech, GxP workflow automation, design control system, audit-ready document control, regulatory compliance platform.